
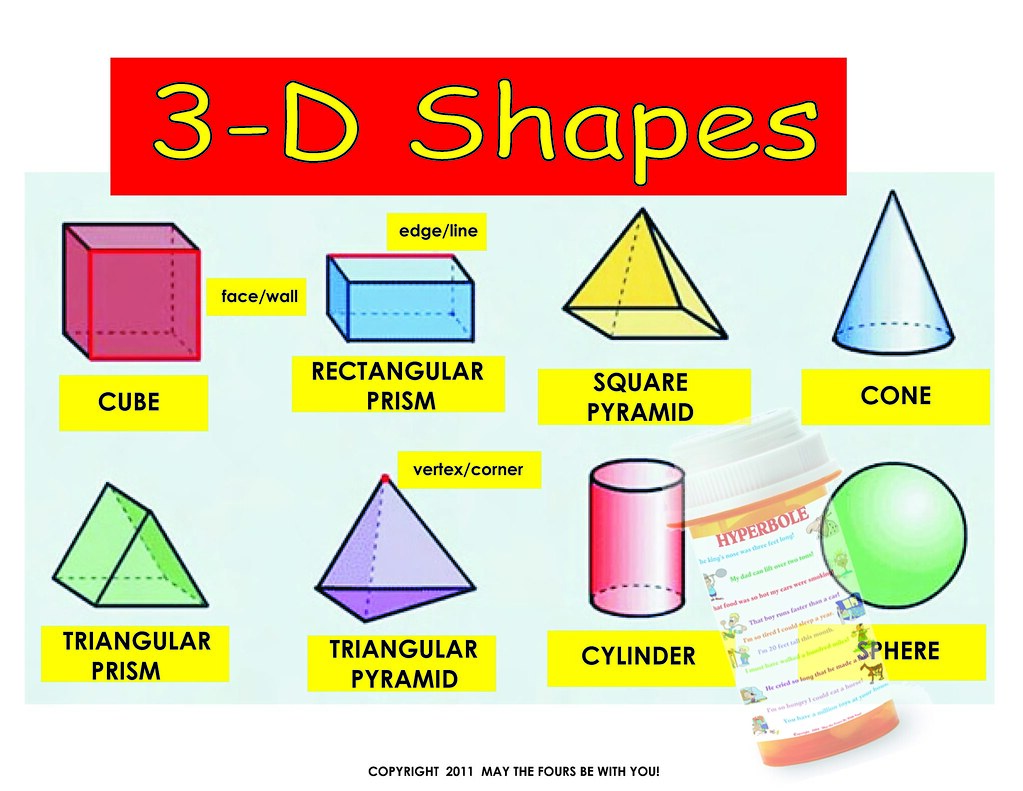
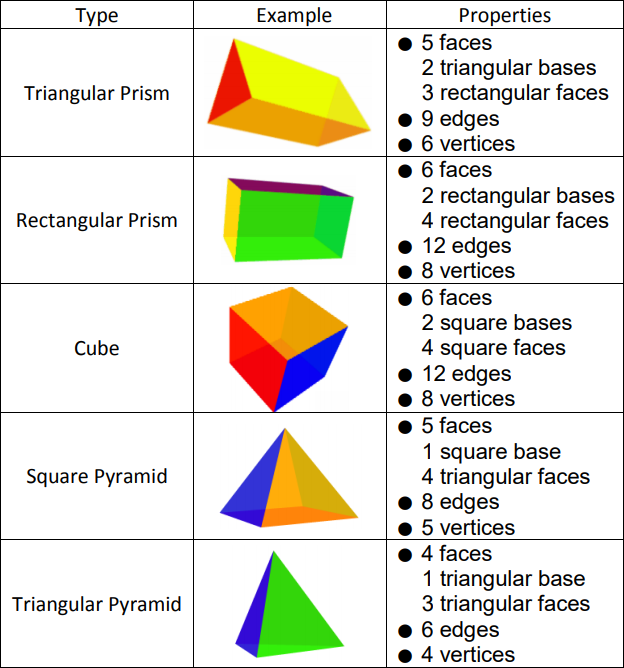
Domains in front of other domains are shaded.There are many 3 dimensional shapes (3D shapes) that have different bases, volumes, and surface areas. Single bond domains are blue, multiple bond domains are green, and lone pair domains are red. The electron domain model for predicting molecular shapes. Multiple bonds also take up more space than single bonds, but still act like a single domain, because they are held by the same two atoms. So we can expect bond angles are a little smaller than we might think, because lone pairs take up more space and push the bonds closer together. Lone pairs are a little flatter and take up more space, because there's no other nucleus pulling them away in a specific direction. Bonding pairs are a little narrower because they are attracted to 2 nuclei and try to stay between them. You can picture each pair of electrons as being a big soft balloon, as in the diagram.
We can think of the core electrons having their own area closer to the nucleus, although it's actually a little more complicated than this.) For the same reason, the different parts of a molecule usually spread out also, so the different parts don't get too close and bump their electron pairs. (We only worry about the valence electrons. The area occupied by a lone pair or a bond is called a domain. The result is that the bonding and non-bonding electron pairs each take up their own area and try to stay as far away from each other as they can. The 2 paired electrons with opposite spin that make up a bond can be in the same general area between the bonding nuclei (although they will still try to avoid each other within this area), but electrons with the same spin really have to give each other space. The basic idea is that while all electrons repel each other, electrons with the same spin repel each other even more.
Lewis structures are a great way to predict the shapes of molecules. Predicting Molecular Shapes (Bond Angles) Thus, these 3 compounds are molecular gases. This is only more true for PF 5 and SF 6, in which the F atoms surround the central atom even more completely. When the molecules pack in the solid, the F atoms touch other F atoms, not Si atoms, and are not attracted much at all. In SiF 4, however, each Si is surrounded by 4 "fluoride ions" (because radius decreases across the periodic table) which naturally makes a tetrahedron, and thus clearly separated molecules. All the ion-ion attractions must be loosened to melt the solid, which requires high temperature. Each cation is surrounded by 6 fluoride ions, and each fluoride ion is surrounded by 6, 3, or 2 cations (depending on the formula). Instead of clearly-defined molecules, there is an alternating lattice of positive and negative ions. In the first 3, the structure is typical of ionic compounds. We can explain the change better using molecular structure than bond type. You might take this as the change from an ionic compound to a covalent compound, but the difference in electronegativity between Al and Si isn't that large, and both are much less electronegative than F. Notice that there is an abrupt change in the melt point between AlF 3and SiF 4. Shapes of molecules can have other effects as well. In general, shapes of molecules will influence how they react, because they determine polarity (which can help pull molecules together to react) and fit (whether the reactive parts can get close to each other).
If it were linear, it would not be a great solvent for polar compounds, and all life would be completely different. For instance, water has a dipole moment because it is bent, not linear. \)ģD structures are important because they can have big effects on the properties of molecules.


 0 kommentar(er)
0 kommentar(er)
